The Woolcock Institute of Medical Research
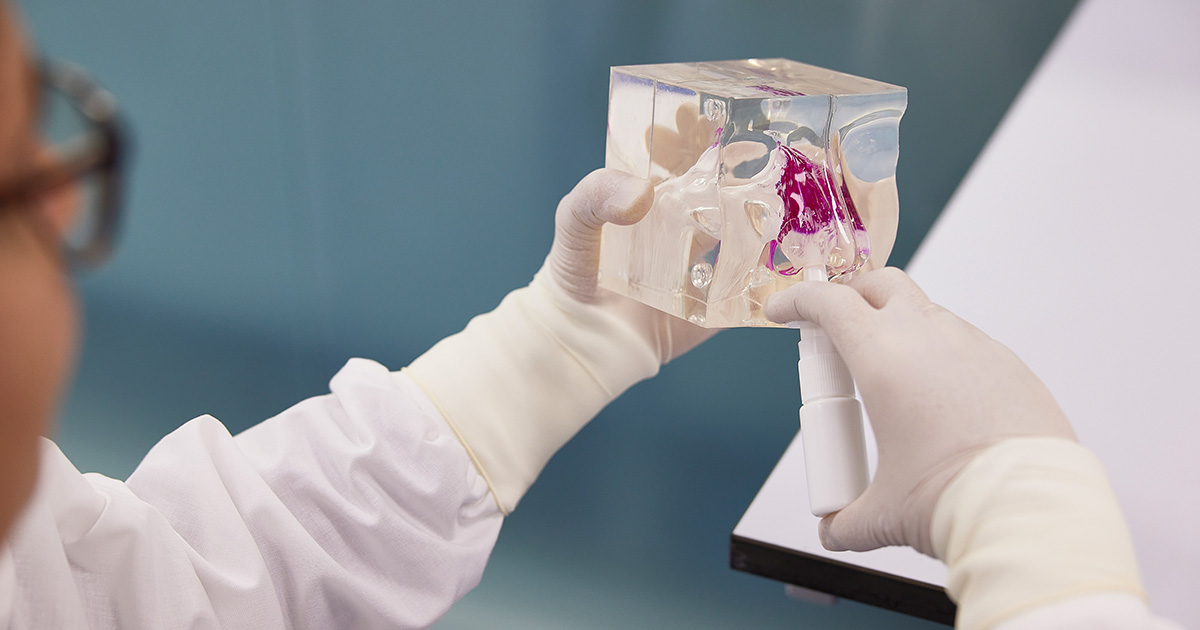
In vitro revolution
It’s been an exciting year for Professor Daniela Traini and for Respiratory Technology, her field of research.
At the annual DDL (Drug Delivery to the Lung) conference held in December last year she received a career award and delivered the keynote speech.
Professor Traini’s topic was the ramifications of legislation approved by the USA Congress in 2022. This significant piece of legislation was enacted to modernise and streamline the drug development approval process. Regulators will now allow alternative non-clinical tests in addition to or instead of animal testing.
That’s exciting because it's a domain where she and her team, with expertise spanning bioengineering, pharmaceutical technology, cell biology, and manufacturing formulation, have dedicated years of effort.
“This change was done with the simple removal of one word from an 85-year-old American regulation from a very conservative agency: by replacing the word ‘animal’ with ‘non-clinical tests’. What the FDA has said with this change is that if you have good in vitro data from a sound model that is equally representative compared with data from animals and has been validated and proven to give similar or superior results, we will accept it or at least we will consider it. This amendment sends a signal of change to the whole field of drug development and could mean a major shift away from current animal testing.” Professor Traini explains.
GOING FOR GOLD
“Now, industry is looking seriously at in vitro methods to limit or avoid animal testing because, first of all it is ethically challenging, secondly, it’s very expensive, and sometimes the animal model is not actually representative of what happens in humans so it’s not always very translatable anyway.”
The Respiratory Technology research group is working on a comprehensive model of the respiratory tract. They have a head start with the Woolcock Lung, first developed by fellow research leader Dr Ong more than three years ago.
“We have to provide the evidence that our results are comparable to results obtained in animals. It's a matter of little by little becoming the gold standard. It doesn't happen quickly, but we are making strides in this field.”
“The end game is to create in vitro models that are accurate enough for there to be less animal testing. That would be fantastic, but it’s a long game – and it may take some years before the agency accepts these models.”
Professor Traini is used to playing a long game. When she started working as a pharmaceutical technologist in 2005, most medical research was tied closely to academia and government funding. That world, she says, is now changing.
Want to stay up to date with our research on sleep and respiratory conditions?
Sign up to our monthly newsletter
BRIDGING THE DIVIDE
“We’ve always been entrepreneurial, and we’ve always worked with industry. I think that's the future; you must diversify your funding stream. Government has changed, the NSW government is pushing for commercialisation of projects and courses on innovation. Our move to Macquarie University is important for that too. They’re keen to help, they’re relatively small and agile and have that business mindset.”
Professor Traini describes much of what she does as a bridge between science and medicine.
“We rely on scientists conducting fundamental research and clinicians presenting issues, yet without pharmaceutical technologists they cannot bring solutions to fruition. We are the ones who resolve projects and address challenges.”
The team are currently working on a project for a new green propellant for metered dose inhalers. That’s no easy task because all the hardware (the valves, the elastomers that are used to work with the propeller) may not work with the new propellants, the vapour pressure is different and consequently the aerosol plume that comes out is different. Particle droplet size is also different.
“There are layers of problems. People think it’s just a matter of switching one thing for another but it’s not as simple as it seems.”
TARGETING THE BRAIN
What excites her about the next few years is the potential for nasal delivery for the treatment of neurodegenerative disease. It’s a project they’re working on with Macquarie’s Dementia Centre, led by Dr Wong.
“The brain is difficult to reach. Current treatments use very large doses systemically – they’re expensive, they have side effects which are significant when you consider the small amount of the drug that gets where you want it to.”
“You have to find a way to cross the blood-brain barrier and the olfactory bulb is a potential access point. You can then use smaller doses of the treatment and target specifically where you want to go without going anywhere else. So, it's a much more targeted approach.”
“We're still in the phase of trying to understand how to reach the brain via the nose. We think we know, but there are still lots of unknowns – what particle size is the best, what surface charge is needed, what device is the best and what area of the nose to target to go to the brain.”
“If it works, it will be revolutionary.”










